Glutathione S-transferase (GST) is a family of enzymes that play a key role in the detoxification of various toxic and carcinogenic compounds in the body.
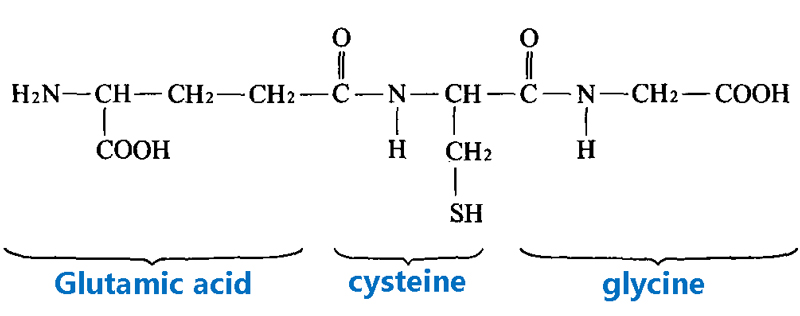
GSTs catalyze the conjugation of glutathione (GSH), a tripeptide composed of glycine, cysteine, and glutamate, to electrophilic substrates, such as halogenated hydrocarbons, epoxides, aldehydes, and nitroaromatics. This reaction reduces the reactivity of the substrates and makes them more soluble and easier to excrete.
GSTs also modulate the activity of some hormones, cytokines, and transcription factors by altering their redox state or binding to them.
GSTs are involved in many physiological processes, such as cellular protection against oxidative stress, inflammation, apoptosis, and cell signaling.
GSTs are widely distributed in various tissues and organs of animals, plants, fungi, and bacteria.
They are classified into three major superfamilies:
cytosolic, mitochondrial, and microsomal (also known as membrane-associated proteins in eicosanoid and glutathione metabolism or MAPEG).
The cytosolic GSTs are further divided into 13 classes based on their amino acid sequence similarity and substrate specificity:
alpha (A), beta (B), delta (D), epsilon (E), zeta (Z), theta (T), mu (M), nu (N), pi (P), sigma (S), tau (U), phi (F), and omega (O). The mitochondrial GSTs belong to the kappa (K) class, while the MAPEG GSTs comprise six subgroups: I to VI.
The structure of GSTs consists of two identical or homologous subunits, each with a molecular weight of about 25 kDa.
Each subunit has two domains:
an N-terminal thioredoxin-like domain and a C-terminal alpha-helical domain. The active site of GSTs is located at the interface of the two domains and contains a GSH-binding site (G-site) and a substrate-binding site (H-site).
The G-site is highly conserved among different classes of GSTs, while the H-site is more variable and determines the substrate specificity of each GST.
The H-site can accommodate a wide range of electrophilic substrates with different sizes, shapes, and charges.
The mechanism of Glutathione S-transferase involves two steps:
first, the nucleophilic attack of the sulfhydryl group of Glutathione on the electrophilic center of the substrate, forming a thioether intermediate; second, the displacement of the intermediate by a water molecule or another nucleophile, releasing the conjugated product.
The reaction is facilitated by a catalytic residue in the active site, which can be a tyrosine, serine, or cysteine depending on the class of GST.
The reaction is also influenced by several factors, such as pH, temperature, ionic strength, cofactors, inhibitors, and polymorphisms.

GSTs have important implications for human health and disease. GSTs protect the cells from the damage caused by reactive oxygen species (ROS), which are generated during normal metabolism or by exposure to environmental pollutants, drugs, radiation, or infection.
ROS can cause oxidative stress and damage to DNA, proteins, and lipids, leading to mutations, inflammation, aging, and diseases such as cancer, neurodegeneration, cardiovascular disorders, diabetes, and asthma.
GSTs also regulate the levels of some endogenous compounds that have biological effects on cell growth, differentiation, apoptosis, and immune response.
For example, GSTs modulate the levels of prostaglandins, leukotrienes, steroids, thyroid hormones, and nitric oxide.
GSTs are also involved in drug metabolism and resistance. Many drugs are metabolized by GSTs to less active or inactive forms that can be eliminated from the body.
However, some drugs are activated by GSTs to more potent forms that can exert therapeutic effects or cause adverse reactions.
For instance,
cyclophosphamide, a chemotherapeutic agent used to treat various cancers, is activated by GSTA1 to form phosphoramide mustard, which alkylates DNA and induces cell death.
On the other hand, paracetamol (acetaminophen), a widely used analgesic and antipyretic drug, is metabolized by GSTP1 to form N-acetyl-p-benzoquinone imine (NAPQI), which depletes Glutathione and causes hepatotoxicity.
The activity and expression of GSTs vary among individuals due to genetic polymorphisms or environmental factors.
Some polymorphisms result in changes in the amino acid sequence or structure of GSTs that affect their catalytic efficiency or substrate affinity.
Other polymorphisms affect the transcriptional regulation or splicing of GST genes that alter their expression levels or isoform distribution.
These variations can influence the susceptibility or resistance of individuals to various diseases or drugs.
For example,
GSTM1 and GSTT1 null genotypes, which are associated with the absence of functional GSTM1 and GSTT1 enzymes, are linked to increased risk of lung, bladder, and colorectal cancers.
Conversely, GSTP1 Ile105Val polymorphism, which results in a reduced activity of GSTP1 enzyme, is associated with decreased risk of prostate and breast cancers.
In summary,
GSTs are a family of enzymes that catalyze the conjugation of Glutathione to electrophilic substrates for the purpose of detoxification.
GSTs have diverse structures, functions, and roles in various physiological and pathological processes. GSTs are involved in cellular protection against oxidative stress, inflammation, apoptosis, and cell signaling.
GSTs also modulate the metabolism and activity of some hormones, cytokines, transcription factors, and drugs. GSTs are influenced by genetic polymorphisms and environmental factors that affect their activity and expression.
GSTs have important implications for human health and disease, as they can modulate the susceptibility or resistance of individuals to various diseases or drugs.