
- Home
-
Abouts
- Classify
- Technology
- Certification
- FAQ
- Video
- Products
- Service
- News
- Contact






Brand: GSHWORLD
Quantity: In Stock
API Citicoline 99% Raw Powder with Bottom Price Citicoline Sodium Pharmaceutical Intermediate, Find Details and Price about Pharmaceutical Medicine from API Citicoline 99% Raw Powder with Bottom Price Citicoline Sodium Pharmaceutical Intermediate - Anhui GSH Bio-Technology Co., Ltd.
Samples: US$ 0/Bag
Supplier Present: GSH Bio-Tech global pioneer in enzymatic catalytic ATP regeneration technology, committed to providing customers with better and more environmentally friendly products and services.
We also provide item sourcing and flight consolidation solutions. We have now our very own manufacturing facility and sourcing place of work. We could provide you with nearly every kind of merchandise associated to our merchandise variety for China Factory - Manufactur standard China Citicoline 99% Raw Powder/CAS No. 33818-15-4/Citicoline Sodium Pharmaceutical Intermediate, The product will supply to all over the world, such as: India, Irish, Italy, Our company insists on the purpose of "takes service priority for standard, quality guarantee for the brand, do business in good faith, to provide professional, rapid, accurate and timely service for you". We welcome old and new customers to negotiate with us. We will serve you with all sincerity!
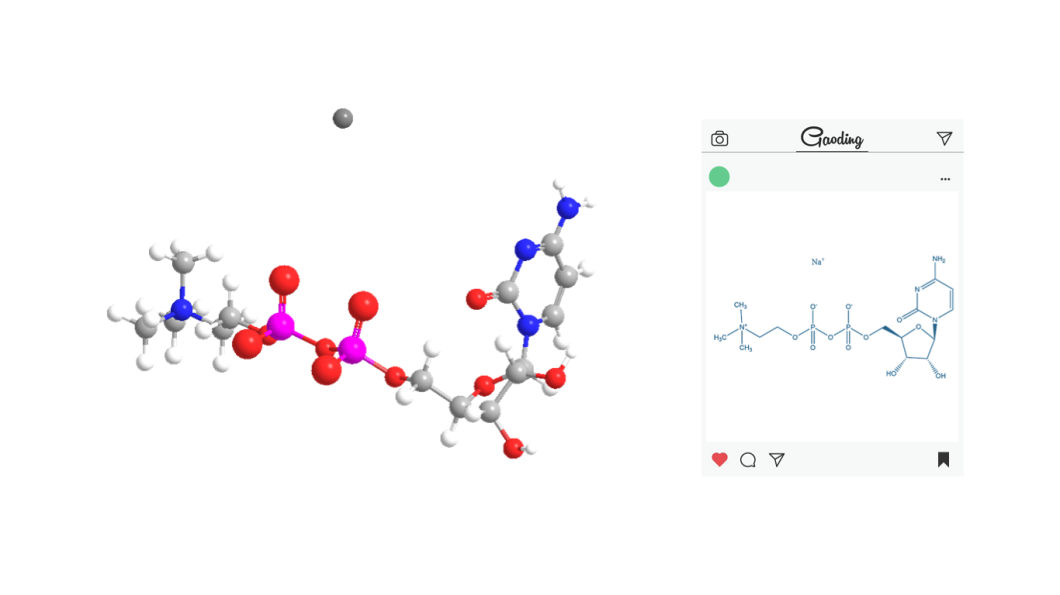
Citicoline sodium (CDPC) is an endogenous nucleoside naturally produced in the body. The repair of nerve cell membranes requires alarge amount of citicoline sodium. It has the functions of repairing brain damage and anti-oxidation, and has been widely used in clinical.
| Model NO. | Citicoline Sodium |
| Shelf Life | 2 Years |
| CAS No. | 33818-15-4 |
| Storage Conditions | Shading, Sealed Storage |
| Description | a White Crystalline or Crystalline Powder, Odorles |
| Clarity, Color Ofsolution | Clear, Coloress |
| Assay(HPLC) | 98 0% to 102 0% |
| Transport Package | 25kg/Drum |
| Specification | 99% |
| Trademark | GSH |
| Origin | China |
| HS Code | 29420090 |
| Production Capacity | 200tons/Year |
Citicoline sodium(C14H25N4NaO11P2) | |
CAS | 33818-15-4 |
Appearance | White powder |
Assay | ≥98.0% |
Loss on drying | Not more than 1% |
Heavy Metal | Not more than 10ppm |
Application | Raw material,medicine |
Shelf Life | 24 months when properly stored. |
Citicoline is amultimodal agent that has demonstrated neuroprotective and neuroregenerative effects in various experimental and clinical disorders of the central nervous system, including acute and chronic cerebral ischemia, cerebral hemorrhage, and global cerebral hypoxia. It provides neuroprotection by attenuating glutamate excitotoxicity, oxidative stress, apoptosis, and blood-brain barrier dysfunction.
In recent years, in addition to being used in brain surgery and traumatic brain injury, it is also used in the auxiliary treatment of function and consciousness, tremor paralysis, tinnitus and nervous deafness, glaucoma and amblyopia caused by acute injury of the central nervous system.
Citicoline may also act as an adjuvant therapy and prevent cognitive decline and other neurological complications associated withdisease 2019.
CDPC can be administered intravenously, intramuscularly or orally: after administration, citicoline sodium is catabolized relatively quickly and is the source of choline in the blood. After oral administration, citicoline sodium is rapidly absorbed and then hydrolyzed into choline and cytidine in the intestinal wall and liver; thus, in addition to providing metabolic precursors of phospholipids, it also enters the synthesis pathways of nucleic acids, proteins and acetylcholine.
Today, citicoline is available as an oral, intravenous infusion, intramuscular injection, and eye drops.




We have QC team to gurantee the quality. All products must have been inspected before delivery. we do inline inspection and final inspection.
1.All raw material checked once it arrive our factory.
2.All details checked during production.
3.All packing details checked during production.
4.All production quality and packing checked on final inspection after finished.
Citicoline Sodium manufacturer / China Citicoline manufacturers / Citicoline Sodium Factory / Citicoline suppliers
Our company since its inception, always regards product quality as enterprise life, continuously improve production technology, improve product quality and continuously strengthen enterprise total quality management, in strict accordance with the national standard ISO 9001:2000 for Citicoline 99% Raw Powder , CDPC-Na , Citicoline Sodium Pharmaceutical Intermediate , To significantly increase our service quality, our corporation imports a large number of overseas advanced devices. Welcome clientele from home and overseas to connect with and inquire!
PREVIOUS: Citicoline Sodium with Top Quality 33818-15-4 Citicoline From Factory Stock
NEXT: Glutathione Powder Food/Cosmetic Grade for Supplement 70-18-8

Material world
Multi - warehouse fast delivery

Return all
Rest assured shopping return worry

Delicate service
Exquisite service and after-sales guarantee

With reduced activity
Contact us to get a discount now