Enzymes on Surfaces of Immune Cells Deplete NMN and NAD+ During Aging
The vital compound nicotinamide adenine dinucleotide (NAD+) binds to enzymes in cells involved in reactions that generate energy for the body and enzymes called PARPs and sirtuins to maintain the health of cells. Levels of NAD+ decline with age, which contributes to the dysfunction of life-sustaining chemical reactions (metabolism) and reduced overall physical fitness, but how this happens isn’t clear. Scientists have proposed multiple mechanisms for how the levels of NAD+ in cells decline during aging: a reduction in levels of an enzyme in cells involved in the synthesis of NAD+ called NAMPT, an increase in consumption of NAD+ by a DNA repair enzyme PARP1, and an increase in the levels of an enzyme called CD38 that consumes NAD+. The inhibition of CD38 preserves NAD+ tissue levels during chronological aging, yet several aspects of CD38’s role in consuming NAD+ have remained unclear in the aging research field, such as which cells possess CD38 during aging, what drives increased levels of CD38, and what role does CD38 play in regulating levels of NAD+ and its precursor molecules.
Recently, Chini and colleagues from the Mayo Clinic in Rochester, Minnesota, published a study in Nature Metabolism indicating that the presentation of CD38 on the exterior of immune cells increases with age due to inflammation resulting from aging processes and that the accumulation of CD38 leads to decreased levels of NAD+ and its precursor molecule, Nicotinamide Mononucleotide (NMN).
Evidence indicating CD38 enzymes from immune cell surfaces reduce NAD+ levels came from experiments where the scientists transferred bone marrow between mice. Genetically altered mice without CD38 enzymes received bone marrow from normal mice because the marrow from normal mice contained stem cells that can restore the presence of cells with CD38 enzymes. In tissues that typically contain high levels of immune cells like the intestine and spleen, NAD+ levels decreased as CD38 activity increased in the mice without CD38 that had CD38 activity restored with bone marrow transplantation, suggesting that CD38 diminishes NAD+ levels.
The results of the study also suggested that inflammation induced the accumulation of immune cells with CD38, leading to decreased NAD+ and NMN levels. Treatment of fat tissue called white adipose tissue (WAT) with an inflammation-inducing molecule called lipopolysaccharide led to reductions in NAD+ and NMN levels. An experiment where the scientists again transferred bone marrow from normal mice to mice without CD38 showed that inflammation from lipopolysaccharide administration exacerbated the effects of CD38 in reducing NAD+ and NMN levels. This shows that inflammation can increase CD38’s consumption of NAD+ and NMN.
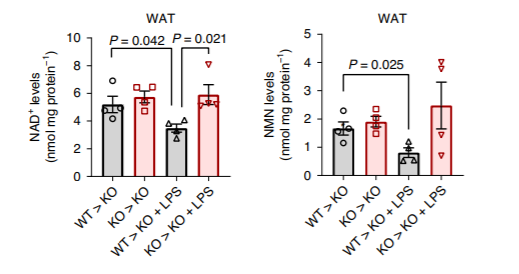
The study went on to demonstrate that immune cells that presented CD38 accumulated in tissues with excessive aged cells that secrete soluble factors that are associated with aged tissue. Furthermore, the accumulation of CD38 could be induced with these soluble factors associated with aging processes. The researchers took a solution from older cells that had displayed signs of aging called senescence–when cells stop proliferating–and added it to white adipose tissue, which caused tissue NAD+ decline. This data indicated that soluble factors associated with aged cells cause diminished NAD+ levels.
What’s more, the researchers demonstrated that CD38 enzyme activity is present on the surface of immune cells that secrete molecules to stimulate immune responses called M1 macrophages and that the CD38 enzymes on these macrophages regulate the availability of the NAD+ precursor NMN. The scientists treated M1 macrophages with lipopolysaccharide to induce inflammation and then with NMN. They observed that NMN levels decreased in the solution containing the macrophages following the treatment with lipopolysaccharide, but this effect was reversion with the CD38 inhibitor Ab68. These results indicated that inflammation facilitated M1 macrophage consumption of NMN via CD38.
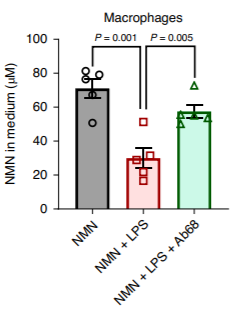
“One key finding from our studies is that NMN is one of the major substrates for CD38,” stated the scientists in their publication with reference to CD38’s consumption of NMN. They go on to say that the NMN-consuming activity of CD38 regulates NAD+ levels by controlling NMN availability.
Along the lines of CD38 as a source of NMN depletion, the scientists found that giving aged mice the CD38 inhibitor Ab68 augmented the NAD+-boosting effects of NMN. These NAD+ boosting effects were significantly enhanced in older mice compared to younger mice due to the natural reductions in cellular NAD+ levels with age. The experiment used to gather this data compared the effects of administering Ab68, NMN, ors Ab68 lpu NMN in three-month-old (young) and 18-month-old (aged) mice.
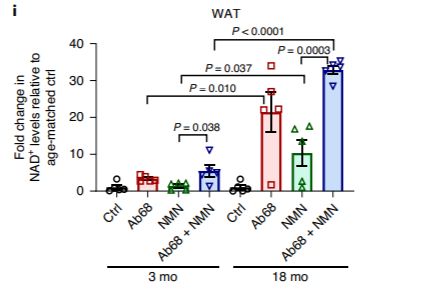
The results of the study show that aged, senescent cells in aged tissues play critical roles in driving the accumulation of immune cells with CD38 enzyme activity, which contributes to age-related NAD+ level decline. How NAD+ levels decrease with age is complex, and future studies will need to examine what pathways cells use to reach a better understanding of NAD+ biology in aging, according to the authors. This study offers a glimpse, though, of the progress researchers continue to make in figuring out the cellular mechanisms behind diminished cellular NAD+ with age.
*Special note - This article is for informational purposes only and cannot replace a doctor's treatment diagnosis and advice. It should not be regarded as a recommendation or proof of efficacy of the medical products involved. If it involves disease diagnosis, treatment, and rehabilitation, please be sure to go to a professional medical institution to seek professional advice.
Previous: The Molecules SS-31 and NMN Synergistically Improve Aged Heart Function
Next: Immune Cells Expressing CD38 Drive NAD+ Decline with Age
by GSHWORLD
GSH Bio Tech is China Biological API Manufacturer. China NMN Supplements powder suppliers & best NMN benefits raw material Factory.













