Immune Cells Expressing CD38 Drive NAD+ Decline with Age
An essential molecule for cellular energy generation that sustains life itself called nicotinamide adenine dinucleotide (NAD+) plays critical roles in our overall health. Recent studies have revealed a progressive decline in NAD+ levels with aging in rodents and humans in multiple tissues. The restoration of NAD+ levels with precursors like nicotinamide riboside (NR), nicotinamide (NAM), and Nicotinamide Mononucleotide (NMN) appears to ameliorate several age-related diseases from Alzheimer’s disease to cardiovascular diseases. Such observations have driven research geared toward understanding how and why NAD+ levels decline with age and toward the development of therapeutics against age-related diseases.
Recently, Verdin and colleagues from the Buck Institute for Research on Aging in California published a study in Nature Metabolism that elucidated a molecular mechanism whereby aging drives reductions in cellular NAD+ levels. According to their study, aged cells that no longer proliferate promote NAD+ level decline via the activation of enzymes on the surfaces of activated immune cells called M1 macrophages.
Enzymes found on the surface of all immune cells called CD38 have been shown to consume NAD+, and the levels of this enzyme increase during inflammatory conditions. During aging, chronic low-grade inflammation occurs, called “inflammaging,” a mechanism that scientists believe could lead to age-associated diseases. The increased CD38 levels in inflammatory conditions along with the low-grade inflammation from aging suggest an integration between the immune system and metabolism. “Despite renewed interest in both NAD metabolism and immunometabolism, little is known about how NAD levels are regulated by immune cells and whether NAD levels influence immune-cell function,” stated the researchers in the study.
To find out how macrophages present in tissue contribute to age-associated changes in NAD+ levels, the team isolated macrophages from the bone marrow of mice. The macrophage can exist in three different states, M0, M1, and M2, which are defined as being not activated, activated, and alternatively activated, meaning they had a different means of function than M1 macrophages upon activation, respectively. They showed that M1 macrophages from the bone marrow have surface proteins called CD38 enzymes that degrade NAD+. The team measured protein levels of enzymes that degrade NAD+ on different types of macrophages from mice as well. Their data indicated that only M1 macrophages and not the M0 and M2 macrophages contained the CD38 enzyme known to degrade NAD+ and that the CD38 enzyme is a significant source of NAD+ consumption for M1 macrophages.
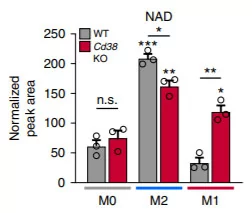
(Covarrubias et al., 2020 | Nature Metabolism) Without CD38, immune cells in their activated state called M1 macrophages have significantly higher NAD+ levels. M0 denotes inactive macrophages, M2 denotes macrophages in an alternatively activated state, and M1 denotes macrophages in an activated state. According to the graph, the cellular solution containing M1 macrophages without CD38 has significantly more NAD+ in comparison to normal macrophages (WT). This effect does not occur with M2 macrophages without CD38 enzymes.
The team of researchers also showed that aged cells release chemicals to trigger higher levels of CD38 in M1 macrophages, a process that depletes NAD+ levels. When the team induced cellular aging with a chemical called doxo or with radiation treatment and collected the solution surrounding these cells, the mouse cells ceased to replicate and grow and release these chemicals associated with aging. They incubated M1 macrophages with the solution from the aged cells for 24 hours and found higher levels of CD38 on the surfaces of the macrophages following the incubation. These findings indicate that the chemicals related to cellular aging induce higher expression of CD38 on M1 macrophages leading to NAD+ consumption.
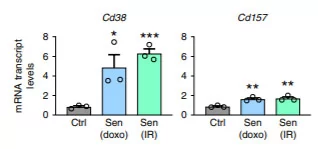
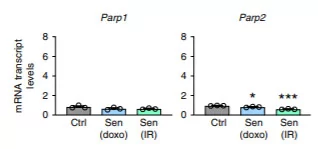
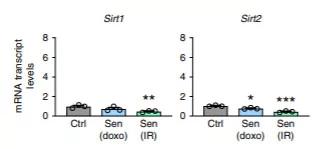
(Covarrubias et al., 2020 | Nature Metabolism) Cellular aging with diminished cell proliferation results in higher expression of CD38 but not other NAD+ consuming proteins. The chemicals doxo along with radiation were used to induce cellular aging, and M1 macrophages were incubated in the solution containing cellular chemicals associated with aging. The graph on the left shows increased expression of CD38 with doxo or radiation compared to the other NAD+ consuming proteins, CD157, PARP1, PARP2, SIRT1, and SIRT2.
“We show that ageing-related inflammation drives the polarization of tissue-resident macrophages to an M1-like state characterized by increased expression of CD38 and enhanced NAD+ consumption,” stated the researchers in reference to their findings. The cross-talk that the team discovered between aged cells and immune cells that modulate tissue NAD+ levels could provide a therapeutic route to target age-associated inflammation by helping to restore NAD+ levels and metabolic function in aging individuals.
*Special note - This article is for informational purposes only and cannot replace a doctor's treatment diagnosis and advice. It should not be regarded as a recommendation or proof of efficacy of the medical products involved. If it involves disease diagnosis, treatment, and rehabilitation, please be sure to go to a professional medical institution to seek professional advice.
PREVIOUS: Enzymes on Surfaces of Immune Cells Deplete NMN and NAD+ During Aging
NEXT: Nicotinamide Mononucleotide (NMN): Benefits, Side Effects & Dosage
by GSHWORLD
GSHWORLD is China Biological API Manufacturer. China NAD+ Supplements powder suppliers & best NAD+ benefits raw material Factory.














